Abstract
Background: Although, post-transplant Cyclophosfamide (PT-Cy) is currently spreading as GvHD prevention, the standard GvHD prophylaxis in unrelated haematopoietic stem cell allogeneic transplant (HSCT) series is still represented by Methotrexate (MTX), a CNI and Anti-thymoglobuline (ATG) combination. The dose of MTX (3 or 4 doses), in association with ATG and CNI remains a matter of debate.
Aim: The Gruppo Italiano Trapianto di Cellule Staminali e Terapie Cellulari (GITMO) analyzed 568 patients who received unrelated (UD) 10/10 HLA matched HSCT and GvHD prophylaxis based on a combination of cyclosporin (CSA), ATG and Methotrexate.
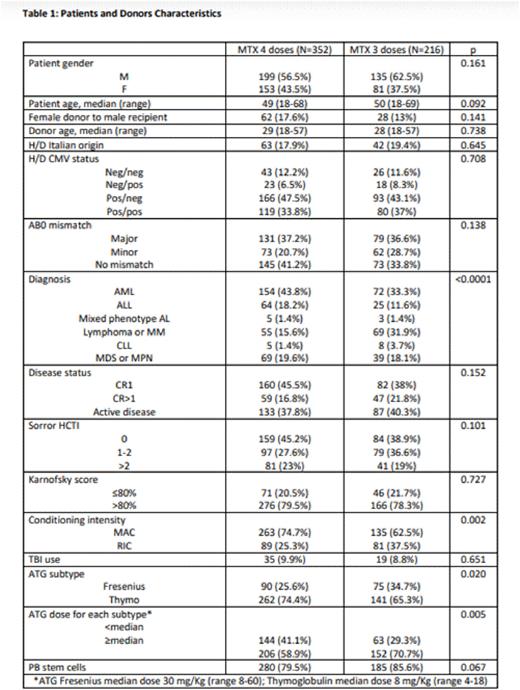
Methods: Patients were grafted in GITMO centers between 2012 to 2015 and GvHD prophylaxis was the same for all patients (ATG-MTX-CSA). The median dose for ATG Fresenius and Thymoglobulin was respectively 30 mg/kg (range 8-60) and 8 mg/Kg (range 4-18); 216 patients received 3 doses of MTX and 352 4 doses. Patient characteristics based on the two groups (MTX 4 and MTX 3 doses) are detailed in table 1. Main differences between the groups concerned conditioning intensity (myeloablative in 75% and 63% for MTX4 and MTX3 respectively, p 0.002), ATG dose (above the median in 59% of MTX4 and in 71% of MTX3 patients, p 0.005), and diagnosis (acute leukemias in 63% of MTX4 and 46% of MTX3, p<0.0001). Statystical analysis: The probabilities of DFS, OS and GRFS were estimated with the Kaplan-Meier method. Cumulative incidence (CI) was estimated for engraftment, GvHD, TRM and relapse to accommodate competing risks. Relapse or progression was a competing risk for TRM, and death from any cause was a competing risk for engraftment and relapse. Relapse or progression and death from any causes were competing risks for GvHD. Univariate comparisons of survival curves were made using the log-rank test and Gray's test was used for univariate comparisons of CI functions. The type I error rate was fixed at 0.05. Statistical analyses were performed with the SPSS (SPSS 22 Inc. IBM, Armonk, NY, USA) and R (R Development Core Team, Vienna, Austria) software.
Results In the setting of 10/10 HLA matched unrelated allogeneic HSCT, whose median follow up was 42.5 months (range 3-75), univariate analysis showed that the administration of 3 versus 4 doses of MTX plays a significant favorable impact on the cumulative incidence of engraftment in terms of PMN (94% vs 90%, p=0.04) and PLTS (90% vs 86%, p= 0.04). Moreover, the 3 MTX doses group achieved significant better results in terms of 3-years CI of TRM (15% vs 23%, p=0.03), OS (66% vs 52%, p=0.001), PFS (54% vs 41%, p= 0.001) and GRFS (44% vs 27%, p=0.00001). We built a multivariate Cox model based on the following covariates: patient age (median value), Donor/host gender, Donor/host CMV status, Disease status, HCT-CI Sorror, Karnofsky PS, Conditioning intensity, Source of stem cells (PB vs BM), ATG dose (median value), MTX dose (4 doses vs 3), Center (≥10 transplants/year vs <10 transplants/year), host/donor Italian origin. Multivariate analysis confirmed the significant protective role of 3 MTX doses on grade 3-4 aGvHD (HR 0.41, p=0.03), TRM (HR 0.59, p=0.02), OS (HR 0.59, p=0.001), PFS (HR 0.6, p<0.0001) and GRFS (HR 0.58, p<0.0001). Interestingly, the relapse risk was significantly inferior in patients who received 3 doses of MTX instead of 4 (HR 0.60, p=0.003).
Conclusions: A fourth dose of MTX in patients receiving ATG+ CSA may negatively impact on engraftment, aGvHD, TRM, OS, PFS and GRFS. Our data support the need for a prospective investigation of MTX doses in the context of patients receiving unrelated matched HSCT and ATG+a CNI.
Disclosures
Picardi:Novartis: Honoraria; Jazz: Honoraria; MSD: Honoraria; Amgen: Honoraria; Gillead: Honoraria. Rambaldi:Astellas: Honoraria; Jazz: Honoraria; ABBVIE: Honoraria; Amgen: Honoraria; Kite-Gilead: Honoraria; Novartis: Honoraria; Incyte: Honoraria; Roche: Honoraria; Janssen: Honoraria; Celgene-BMS: Honoraria; Omeros: Honoraria; Pfizer: Honoraria. Angelucci:Novartis: Honoraria; Celgene: Honoraria, Other: Data monitoring committee; Bluebird Bio: Consultancy; Menarini/Stemline: Consultancy; Gilead: Consultancy; Roche: Consultancy; Vertex: Honoraria, Other: Data monitoring committee; Sanofi: Speakers Bureau; Vifopr: Honoraria, Other: Data monitoring committee; Glaxo: Consultancy. Patriarca:Janssen: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Ciceri:Kite Pharma: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal